Australian Asthma Handbook
The National Guidelines for Health Professionals
Cite
Table
| Situation | Total dose per occasion | Administration |
| Cough or wheeze without visibly increased work of breathing | 2 actuations x salbutamol 100 microg/actuation If symptoms do not resolve within a few minutes, give 2 more actuations. | Administer via spacer (and mask, if needed) Add 1 actuation to spacer – child takes 4 breaths in and out of spacer. Immediately repeat for second and subsequent actuations until the recommended number of actuations have been given. |
| Symptoms with increased work of breathing | 6 actuations x salbutamol 100 microg/actuation If symptoms do not resolve within a few minutes, give 6 more actuations and call 000 for an ambulance. If symptoms still do not resolve, or recur within 3 hours, parents/carer should take child to ED or call 000 for an ambulance. |
Table
| Severity of Exacerbations | Frequency of symptoms | ||
| Less often than once every 3 months | At least once every 3 months but not more than once per month | More than once per month | |
Mild Exacerbations quickly* resolve with salbutamol | Not indicated | Consider | Indicated |
Moderate–severe ≥2 exacerbations required ED or oral corticosteroids in past 12 months | Indicated | Indicated | Indicated |
Life-threatening ≥1 exacerbation required hospitalisation or PICU | Indicated | Indicated | Indicated |
Additional information
ED: emergency department; PICU: paediatric intensive care unit; *within a few minutes
Table
| Active ingredient | Total daily dose (microg) | |
| Low | Medium/high | |
| Fluticasone propionate | 100 (50 twice daily) | 200 (100 twice daily) |
Additional information
ICS: inhaled corticosteroid
Medium/high doses should be avoided except under specialist supervision
Usual doses (100 microg per actuation): 2 actuations via pMDI plus spacer (with face mask, if needed). If symptoms do not resolve within a few minutes, use 2 more actuations.
Recommendation type: Consensus recommendation
All children with asthma require a rapid-acting bronchodilator to manage symptoms as they occur, even if the child is also receiving maintenance treatment with ICS. Salbutamol is currently the first-choice reliever for children aged 1–5 years.
This recommendation applies to children with salbutamol-responsive signs/symptoms and a provisional or confirmed diagnosis of preschool asthma. Intermittent wheeze in an otherwise healthy child does not require salbutamol treatment.
In this age group, inhaled medicines should be delivered using a pressurised metered-dose inhaler with a spacer. A mask should be used for children who cannot form a tight mouth seal around the spacer mouthpiece (e.g. younger children and during an acute asthma exacerbation).
The use of nebulisers is not recommended because they can transmit respiratory viruses [Hui 2009] and are unnecessary except in severe acute asthma. Nebulised salbutamol is associated with more systemic adverse effects than pMDIs. In studies of children with acute asthma, higher rates of tremor have been reported among children treated with nebulised salbutamol than salbutamol via pMDI with spacer.[Cates 2013] In older children and adults, the use of nebulisers for SABA is associated with a higher risk of exacerbations than the use of inhalers.[Paris 2008]
Note on the 2025 recommendation: Anti-inflammatory reliever (ICS plus formoterol or ICS plus salbutamol in a single inhaler) is not approved by the TGA for use in children aged 1–5 years. Future Australian asthma handbook guidance may recommend anti-inflammatory reliever in place of salbutamol, depending on the findings of recent and ongoing clinical trials, and on TGA and PBS decisions.
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev 2013; 9: CD000052.
Hui DS, Chow BK, Chu LC, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 2009; 135: 648-654
Paris J, Peterson EL, Wells K, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol 2008; 101: 482-487.
Resources
Royal Children’s Hospital Melbourne’s What is asthma? videos for parents, explaining how to identify wheeze and other signs, and how to correctly use a pMDI with spacer.
National Asthma Council Australia’s video How to use a metered dose inhaler (puffer) with a spacer for children
National Asthma Council Australia’s Spacer use and care
National Asthma Council Australia’s fact sheet on spacers for pressurised metered-dose inhalers
Higher salbutamol doses may be given when the child shows marked increase in work of breathing, e.g. 6 actuations via pMDI plus spacer. If symptoms do not resolve within a few minutes, the dose can be repeated.
More information on assessing and managing acute asthma in primary care or emergency departments.
When calculating canisters per year, allowing for multiple inhalers in use at the same time.
Recommendation type: Consensus recommendation
Consumption of three or more canisters of salbutamol in a year indicates that the child’s asthma is poorly controlled.
A large cohort study reported that in children aged 1–5 years prescription of three or more SABA canisters per year was associated with at least double the risk of subsequent exacerbations, compared with lower SABA prescribing.[Morgan 2023]
Morgan A, Maslova E, Kallis C, et al. Short-acting β2-agonists and exacerbations in children with asthma in England: SABINA Junior. ERJ Open Res 2023; 9: 00571-2022.
If symptoms are frequent (daytime symptoms twice per week or more, or night-time symptoms twice per month or more), symptoms are restricting activity or sleep, or the child has a history of 2 or more exacerbations treated with systemic corticosteroids, begin a treatment trial with daily maintenance low-dose ICS, in addition to salbutamol as needed.
Trial low-dose ICS for approximately 3 months then review clinical response:
Recommendation type: Consensus recommendation
Prevention of exacerbations requiring systemic corticosteroid treatment is a key goal of asthma management. The principal strategy for reducing the risk of exacerbations is treatment with inhaled corticosteroids.
The use of multiple short courses of oral corticosteroids to manage asthma exacerbations in children is associated with a dose-dependent reduction in bone mineral accretion and increased risk for osteopenia.[Kelly 2008] In adults, short courses of oral corticosteroids to manage asthma exacerbations in adults are associated with increased lifetime risk of osteoporosis, pneumonia, cardiovascular or cerebrovascular diseases, cataract, sleep apnoea, renal impairment, depression/anxiety, type 2 diabetes, and weight gain.[Price 2018]
Note on the 2025 recommendation: Anti-inflammatory reliever (ICS plus formoterol or ICS plus salbutamol in a single inhaler) is not approved by the TGA for use in children aged 1–5 years. Future Australian asthma handbook guidance may recommend anti-inflammatory reliever in place of salbutamol, depending on the findings of clinical trials now underway and on TGA and PBS decisions.
Delivery
Most children younger than 5 years cannot use dry powder inhalers correctly because they cannot achieve sufficient inspiratory flow to activate the device.[Kuek 2024]
Efficacy
Daily ICS treatment reduces rates of symptoms and exacerbations in preschool children with recurrent wheeze due to asthma.[Kaiser 2016, Castro-Rodriguez 2009]
ICS is more effective than montelukast in improving symptom control and reducing exacerbation rates.[Castro-Rodriguez 2018]
Safety
At recommended doses, ICSs are generally well tolerated in children.[Rachelefsky 2009; Kapadio 2016]
The use of a spacers with pMDIs reduces oropharyngeal drug deposition and therefore reduces the risk of local adverse effects (e.g. candidiasis and dysphonia) with ICS.[Lavorini 2020]
Topical effects of ICS can also be reduced by mouth-rinsing and spitting after inhaling. Immediate quick mouth-rinsing removes more residual medicine in the mouth than delayed rinsing.[Yokoyama 2007]
ICS-related systemic adverse effects in children include suppression of the hypothalamic-pituitary-adrenal (HPA) axis (rare),[Kapadio 2016] short-term linear growth suppression, clinically non-significant effects on bone mineral density, and dose-dependent effects on glucose metabolism.[Kapadio 2016]
A review of long-term clinical trials of recommended doses of inhaled corticosteroids in children found little or no effect on measures of HPA axis function over 12 to 36 months follow-up, and no clinically significant effects on bone mineral density.[Pedersen 2006]
Regular use of ICS in children before puberty is associated with an average reduction of 0.48 cm/year in linear growth rate in the first year of treatment, after which less effect is seen. Growth suppression depends on the dose.[Axelsson 2019]
Uncontrolled asthma also reduces children’s growth and final adult height.[Pedersen 2001]
Pausing ICS treatment to reassess symptom status
Spontaneous remission of preschool asthma may occur. Therefore, the need to continue ICS should be repeatedly reviewed in preschool children to avoid unnecessary medication.
Axelsson I, Naumburg E, Prietsch SO, Zhang L. Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database Syst Rev 2019; 6: CD010126.
Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics 2009; 123: e519-25.
Castro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: A systematic review. Pediatr Pulmonol 2018; 53: 1670-1677.
Kaiser SV, Huynh T, Bacharier LB, et al. preventing exacerbations in preschoolers with recurrent wheeze: a meta-analysis. Pediatrics 2016; 137: e20154496.
Kelly HW, Van Natta ML, Covar RA, et al. Effect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the childhood Asthma Management Program (CAMP) study. Pediatrics 2008; 122: e53-e61.
Kuek SL, Wong NX, Dalziel S, et al. Dry-powder inhaler use in primary school-aged children with asthma: a systematic review. ERJ Open Res 2024; 10: 00455-2024.
Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function, and cold air and methacholine responsiveness in 2- to 5-year-old asthmatic children. Am J Respir Crit Care Med 2000; 162: 1500-1506.
Pedersen S. Do inhaled corticosteroids inhibit growth in children? Am J Respir Crit Care Med 2001; 164: 521-35.
Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193-204.
The optimal duration of treatment trial depends on predictable seasonal fluctuation in exacerbations. Avoid stopping at a time when respiratory viruses are prevalent.
Recommendation type: Consensus recommendation
The use of nebulisers is not recommended. Delivery of inhaled medicines by nebuliser is unnecessary,[Cates 2013] except in some patients with severe acute asthma unable to breathe through a spacer.
Nebulisers may transmit respiratory viruses.[Hui 2009]
In children and adults, the use of nebulisers for SABA is associated with a higher risk of exacerbations than the use of inhalers.[Paris 2008]
Nebulised salbutamol is associated with more systemic adverse effects than pMDIs. In studies of children with acute asthma, higher rates of tremor have been reported among children treated with nebulised salbutamol than salbutamol via pMDI with spacer.[Cates 2013] Nebulised ICS can damage the child’s eyes if the face mask is not well fitted. Nebulised ICS can cause rash and atrophy of skin around the nose and mouth.[GINA 2025]
The use of home nebulisers could also lead to delays in treatment, compared with the prompt use of readily transportable pMDIs anywhere outside the home, whenever the child has asthma symptoms.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Hui DS, Chow BK, Chu LC, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 2009; 135: 648-654
Paris J, Peterson EL, Wells K, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol 2008; 101: 482-487.
National Asthma Council Australia Nebuliser use and care
Recommendation type: Adapted from GINA
Oral bronchodilators are not recommended for any age group. Oral salbutamol has a longer onset of action and a higher rate of adverse effects than inhaled salbutamol.[GINA 2025]
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Recommendation type: Adapted from GINA
Oral bronchodilators are not recommended for any age group.[GINA 2025]
Theophylline is less effective than inhaled bronchodilators in reducing symptoms and increasing lung function, and adverse effects are common.[Morali 2001, ALAACRC 2007, Barnes 2013]
American Lung Association Asthma Clinical Research Centers. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med 2007; 175: 235-242.
Barnes PJ. Theophylline. Am J Respir Crit Care Med 2013; 188: 901-6.
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Morali T, Yilmaz A, Erkan F, et al. Efficacy of inhaled budesonide and oral theophylline in asthmatic subjects. J Asthma 2001; 38: 673-9.
Make sure parents understand how to follow the instructions.
Advise parents to give a copy to the child’s preschool/day care and any other carers.
Recommendation type: consensus recommendation
Every child with asthma should have their own written asthma action plan for parents to follow when symptoms worsen.
In children aged 6 years and over, the use of written asthma action plans significantly reduces the rate of visits to acute care facilities, the number of school days missed and night-time waking, and improves symptoms.[Zemek 2008] For children, written asthma action plans that are based on symptoms appear to be more effective than action plans based on peak expiratory flow monitoring.[Zemek 2008]
Zemek RL, Bhogal S, Ducharme FM. Systematic review of randomized controlled trials examining written action plans in children – What is the plan? Arch Pediatr Adolesc Med 2008; 162: 157-63.
National Asthma Council Australia’s Library of asthma action plan templates
Preschools and schools in all Australian states and territories require parents or carers of children with asthma to provide a written asthma action plan stating the child’s known asthma triggers and instructions to be followed if symptoms occur, and emergency procedures in the event of an asthma exacerbation.
Information on asthma action plans for children 1–5 years
Recommendation type: consensus recommendation
Wheezing in children younger than 12 months is usually due to bronchiolitis, which generally does not respond to salbutamol treatment.[Gadomski 2014]
Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2014; 2014: CD001266.
Recommendation type: Consensus recommendation
In children aged 2–3 years with recurrent wheezing, aeroallergen sensitisation is associated with a better clinical response to ICS than to placebo (less systemic corticosteroid use, fewer urgent care and emergency department visits).[Bacherier 2009]
In children aged 1–5 years aeroallergen sensitisation is associated with a better clinical response to ICS than to montelukast (longer time to exacerbations and symptom control).[Fitzpatrick 2016]
Aeroallergen sensitisation is also a predictor of good clinical response to ICS in school-aged children.[Gerald 2015, Szefler 2010]
Bacharier LB, Guilbert TW, Zeiger RS, et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol 2009; 123: 1077-1082.e10825.
Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016; 138: 1608-1618.e12.
Gerald JK, Gerald LB, Vasquez MM, et al. Markers of differential response to inhaled corticosteroid treatment among children with mild persistent asthma [published correction appears in J Allergy Clin Immunol Pract. 2015; 3: 793]. J Allergy Clin Immunol Pract 2015; 3: 540-546.e3.
Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol 2010; 125: 285-294.
If cough has not resolved, stop ICS treatment and reconsider alternative diagnoses.
Recommendation type: Consensus recommendation
In children, chronic cough in the absence of other symptoms/signs is rarely due to asthma.[Marchant 2024]
Cough that is due to asthma typically improves after 2–4 weeks of treatment with ICS. [Marchant 2024]
Australian cough management guidelines recommend against the use of ICS to manage chronic cough in children unless there are specific features to suggest asthma, and that ICS should be ceased if cough does not resolve within 4 weeks.[Marchant 2024]
Marchant JM, Chang AB, Kennedy E, et al. Cough in Children and Adults: Diagnosis, Assessment and Management (CICADA). Summary of an updated position statement on chronic cough in Australia. Med J Aust 2024; 220: 35-45.
Recommendation type: Consensus recommendation
Australian product information – Nasonex aqueous nasal spray 0.05%, alcohol free (mometasone furoate monohydrate). [Revised 16 February 2021] Therapeutic Goods Administration (www.ebs.tga.gov.au)
The Royal Children's Hospital Melbourne’s allergic rhinitis management guide for primary care
National Asthma Council Australia’s Allergic rhinitis treatment planner
Intranasal mometasone furoate spray is TGA-approved for children 3 years and over.[Australian mometasone furoate]
Some nonsedating antihistamines (e.g. desloratadine, loratadine) are approved for use in children aged 5 years and under.
Resources
Explain that:
Medication management
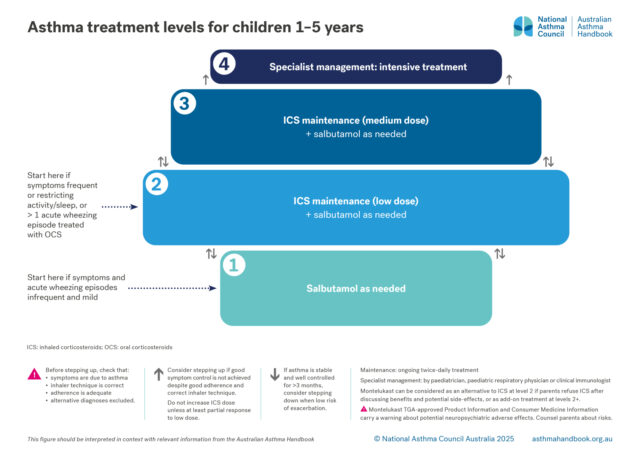
Treatment options for preschool children, classified according to intensity of treatment needed to control asthma…

Principles of management
How to administer inhaled medicines to preschool children.

Principles of management
How to equip and coach parents and carers to manage a preschool child’s asthma, including exacerbations.