Australian Asthma Handbook
The National Guidelines for Health Professionals
Cite
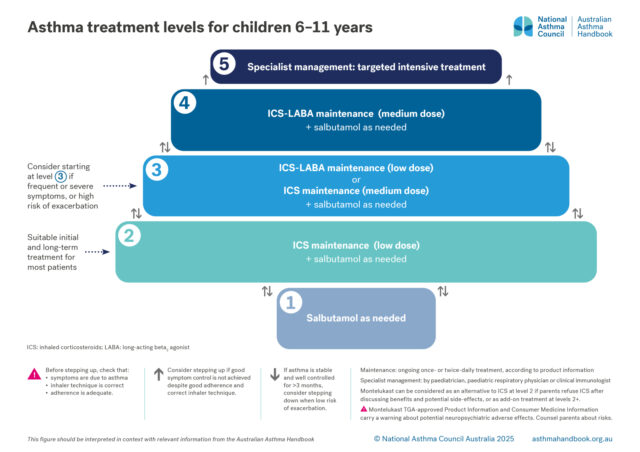
Asthma treatment is adjusted to maintain good control of asthma symptoms and prevent exacerbations, while minimising side-effects. The optimal step for an individual child may change over time. Remission may occur in some children.
There are 5 levels of treatment, from least intensive to most intensive:
Level 3. Maintenance treatment with low-dose ICS-LABA
Alternative: maintenance treatment with medium-dose ICS
Montelukast can be added to ICS-based treatment at levels 3 and above.
Salbutamol as needed
All children with asthma should be treated with salbutamol as needed when symptoms occur, delivered by pMDI plus spacer.
Salbutamol as needed can be considered as the sole asthma treatment for children with mild and infrequent symptoms and no history of a severe exacerbation or risk factors for severe exacerbations.
However, frequent use indicates that the child needs ICS treatment to reduce the risk of exacerbations. Consumption three or more canisters of salbutamol in a year indicates that the child’s asthma is poorly controlled. In a large cohort study reported that, in children aged 6–11 years, prescription of three or more SABA canisters per year was associated with at least double the risk of subsequent exacerbations, compared with lower SABA prescribing. [Morgan 2023]
ICS maintenance treatment
Maintenance treatment with low doses of ICS significantly reduces the risk of asthma exacerbations in children. In a large 3-year clinical trial in children aged 5–10 years with recently diagnosed asthma, maintenance treatment with low-dose ICS (plus SABA as needed) reduced the risk of serious exacerbations by 40%, improved lung function, increased symptom-free days and decreased days lost from school years, compared with SABA only.[Chen 2006]
Most benefit of ICS is seen at low-to-medium doses. High doses achieve small improvements in control but greatly increase the rate of local adverse effects.[Adams 2006]
ICS-LABA maintenance treatment
Maintenance treatment with a combination of an ICS and a LABA in a single inhaler is approved by TGA for use in children ≥4 years.[Australian PI: fluticasone propionate/salmeterol xinafoate]
This option avoids increasing the ICS dose, while achieving at least equal efficacy.[Vaessen-Verberne 2010]
In children with asthma that is not well controlled with low-dose ICS, adding a LABA is more likely to improve asthma than increasing the ICS dose or adding montelukast to low-dose ICS.[Lemanske 2010, Chauhan 2014, Cividini 2023] However, individual responses vary; some children have a better response to other options.[Lemanske 2010]
In children aged 4–11 years, addition of LABA to ICS does not increase risk of exacerbations,[Stempel 2016] contrary to historical concerns.
Safety of ICS in children
At recommended doses, ICSs are generally well tolerated in children.[Rachelefsky 2009; Kapadio 2016]
The use of a spacers with pMDIs reduces oropharyngeal drug deposition and so reduces the risk of local adverse effects (e.g. candidiasis and dysphonia) with ICS.[Lavorini 2020] Topical effects can also be reduced by mouth-rinsing and spitting after inhaling. Immediate quick mouth-rinsing removes more residual medicine in the mouth than delayed rinsing.[Yokoyama 2007]
ICS-related systemic adverse effects in children include suppression of the hypothalamic-pituitary-adrenal (HPA) axis (rare),[Kapadio 2016] short-term linear growth suppression, clinically non-significant effects on bone mineral density, and dose-dependent effects on glucose metabolism.[Kapadio 2016]
A review of long-term clinical trials of recommended doses of inhaled corticosteroids in children found little or no effect on measures of HPA axis function over 12 to 36 months follow-up, and no clinically significant effects on bone mineral density.[Pedersen 2006]
Regular use of ICS in children before puberty is associated with an average reduction of 0.48 cm/year in linear growth rate in the first year of treatment, after which less effect is seen. Growth suppression depends on the dose.[Axelsson 2019]
Uncontrolled asthma also reduces children’s growth and final adult height.[Pedersen 2001]
Montelukast maintenance treatment
Montelukast can be considered for children who cannot use an inhaler, or when parents refuse ICS treatment after discussing benefits and potential adverse effects.
Montelukast monotherapy is generally less effective than ICS or ICS-LABA in preventing asthma exacerbations.[Cividini 2023]
The addition of montelukast to ICS maintenance treatment has not been shown to improve asthma control in children, compared with ICS-LABA.[Chauhan 2014]
Montelukast has been associated with neuropsychiatric disorders in all age groups.[TGA 2018] Depression/anxiety is among the most commonly reported adverse events in children aged 6–11 years.[Aldea Perona 2016] However, a large cohort study of children aged 6 to 17 years treated with montelukast reported no association between use of montelukast and the risk of neuropsychiatric adverse events.[Wintzell 2025]
Tiotropium maintenance treatment
Tiotropium added to ICS-LABA reduces the risk of exacerbations and achieves a small improvement in lung function in children aged 6–11 years.[Rodrigo 2017]
Benefits in children do not appear to depend on asthma phenotype as determined by IgE or eosinophil count.[Szefler 2019]
Adams NP, Jones PW. The dose-response characteristics of inhaled corticosteroids when used to treat asthma: an overview of Cochrane systematic reviews. Respir Med 2006; 100: 1297-1306.
Aldea Perona A, García-Sáiz M, Sanz Álvarez E. Psychiatric disorders and montelukast in children: a disproportionality analysis of the VigiBase®. Drug Saf 2016; 39: 69-78.
Australian Product Information – Pavtide (fluticasone propionate/salmeterol xinafoate) Accuhaler and MDI. [Revised 7 November 2022] Therapeutic Goods Administration (www.ebs.tga.gov.au)
Axelsson I, Naumburg E, Prietsch SO, Zhang L. Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database Syst Rev. 2019; 6: CD010126.
Bisgaard H, Le Roux P, Bjamer D, et al. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest 2006; 130: 1733-1743.
Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev 2014; 1: CD003137.
Chen YZ, Busse WW, Pedersen S, et al. Early intervention of recent onset mild persistent asthma in children aged under 11 yrs: the Steroid Treatment As Regular Therapy in early asthma (START) trial. Pediatr Allergy Immunol 2006; 17 Suppl 17: 7-13.
Cividini S, Sinha I, Donegan S, et al. Best step-up treatments for children with uncontrolled asthma: a systematic review and network meta-analysis of individual participant data. Eur Respir J 2023; 62: 2301011.
Kapadia CR, Nebesio TD, Myers SE, et al; Drugs and Therapeutics Committee of the Pediatric Endocrine Society. Endocrine Effects of Inhaled Corticosteroids in Children. JAMA Pediatr 2016; 170: 163-70.
Lavorini F, Barreto C, van Boven JFM, et al. Spacers and Valved Holding Chambers-The Risk of Switching to Different Chambers. J Allergy Clin Immunol Pract 2020; 8: 1569-1573.
Lemanske R, Mauger D, Sorkness C, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010; 362: 975-985.
Morgan A, Maslova E, Kallis C, et al. Short-acting β2-agonists and exacerbations in children with asthma in England: SABINA Junior. ERJ Open Res 2023; 9: 00571-2022.
Pedersen S. Do inhaled corticosteroids inhibit growth in children? Am J Respir Crit Care Med 2001; 164: 521-35.
Pedersen S. Clinical safety of inhaled corticosteroids for asthma in children: an update of long-term trials. Drug Saf 2006; 29: 599-612.
Rachelefsky G. Inhaled corticosteroids and asthma control in children: assessing impairment and risk. Pediatrics 2009; 123: 353-66.
Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: A systematic review. Pediatr Allergy Immunol 2017; 28: 573-578.
Stempel DA, Szefler SJ, Pedersen S, et al. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med 2016; 375: 840-849.
Szefler SJ, Vogelberg C, Bernstein JA, et al. Tiotropium Is efficacious in 6- to 17-year-olds with asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract 2019; 7: 2286-2295 e2284.
Therapeutic Goods Administration. Montelukast. Safety review [Webpage] 2018.
Vaessen-Verberne AA, van den Berg NJ, van Nierop JC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med 2010; 182: 1221-1227.
Wintzell V, Brenner P, Halldner L, et al. Montelukast use and the risk of neuropsychiatric adverse events in children. JAMA Pediatr 2025; 179: 418-427.
Yokoyama H, Yamamura Y, Ozeki T, et al. Effects of mouth washing procedures on removal of budesonide inhaled by using Turbuhaler. Yakugaku Zasshi 2007; 127: 1245-1249.
Check TGA-approved indications and PBS restrictions before prescribing.
Note on the 2025 recommendation: Maintenance-and-reliever therapy with ICS-formoterol is not approved by TGA for use in the treatment asthma in children younger than 12 years. Future Australian asthma handbook guidance may recommend maintenance-and-reliever therapy for children 6–11 years, depending on the findings of recent and ongoing clinical trials, and on TGA and PBS decisions.
Limited evidence in children 6–11 years suggests that the combination of very low doses of budesonide–formoterol taken as maintenance treatment, with extra doses taken as reliever whenever symptoms occur, is associated with a large reduction in exacerbation rate, compared with the same dose as maintenance treatment with SABA taken as needed for symptoms, and compared with a higher dose of maintenance ICS treatment.[Bisgaard 2006]

Medication management
How to choose optimal starting treatment for children aged 6–11 years.

Medication management
How to step up or step down asthma treatment for a child aged 6–11 years, to maintain symptom control, prevent…