Australian Asthma Handbook
The National Guidelines for Health Professionals
Cite
Table
| Active ingredient | Total daily dose (microg) | ||
| Low | Medium | High | |
| Beclometasone dipropionate (extra-fine particle) | 100–200 | 250–400 | >400 |
| Budesonide | 200–400 | 500–800 | >800 |
| Ciclesonide | 80–160 | 240–320 | >320 |
| Fluticasone furoate | – | 100 | 200 |
| Fluticasone propionate | 100–200 | 250–500 | >500 |
| Mometasone furoate in combination with indacaterol via capsule inhaler | 62.5* | 127.5* | 260* |
| Mometasone furoate in combination with indacaterol and glycopyrronium via capsule inhaler | – | 68* | 136* |
Additional information
ICS: inhaled corticosteroid
The table shows options for low, medium and high doses of each available inhaled corticosteroid (with or without LABA and LAMA) – it does not indicate dose equivalence.
*Doses of mometasone furoate are shown as delivered dose (not metered dose) in line with inhaler labels. For all other products in this table, the inhaler label and table show the metered dose.
Table
| Brand names | Type | Strength (microg)* | Dose (inhalations) | Maximum dose† (inhalations) | Age restriction | PBS code | Authority code | Maximum quantity (2 repeats for all) | Actuations per device |
Rilast Rapihaler Symbicort Rapihaler | pMDI | 100/3 | 2 | 12 per occasion 16 per day 24 in one day temporarily | ≥ 12 years | 12042T | 10482 | 2 | 120 |
| Bufomix Easyhaler | DPI | 200/6 | 1 |
6 per occasion 8 per day 12 in one day temporarily | ≥ 12 years | 14166N 12041R | 10464 10464 | 2 1 | 60 120 |
Rilast Turbuhaler Symbicort Turbuhaler | DPI | 200/6 | ≥ 12 years | 12041R | 10464 | 1 | 120 | ||
| DuoResp Spiromax | DPI | 200/6 | ≥ 18 years | 12029D | 10464 | 1 | 120 |
Additional information
DPI: dry powder inhaler; pMDI: pressurised metered-dose inhaler; *Budesonide dose/formoterol dose; † Maximum doses as stated in TGA-approved product information (daily maximum rarely needed in practice)
TGA-approved indication: Anti-inflammatory reliever therapy is taken as needed for the relief of asthma symptoms when they occur, and as preventative treatment of symptoms in those circumstances recognised by the patient to precipitate an asthma attack. Patients should be advised to always have their anti-inflammatory reliever available for relief of symptoms. If the patient experiences a 3-day period of deteriorating symptoms after taking additional as needed inhalations, the patient should be reassessed for alternative explanations of persisting symptoms. Patient must not be on a concomitant single agent long-acting beta2 agonist. (Check PBS restrictions before prescribing)
Table
| Active ingredients | Brand names (Type) | Strength (microg)* | Dose (inhalations) | Age restriction | PBS codes | Authority code | Maximum quantity (5 repeats for all) | Actuations per device | ||
| Maintenance | Reliever | Maximum‡ | ||||||||
| Beclometasone dipropionate-formoterol | Fostair (pMDI) | 100/6 | 1 twice daily | 1 | 6 per occasion 8 per day | 18 years | 12183F 14376P† | 15469 15599 | 1 2 | 120 |
| Budesonide- formoterol | Rilast Rapihaler Symbicort Rapihaler (pMDI) | 100/3 | 2 twice daily (may increase to 4 twice daily) | 2 | 12 per occasion 16 per day 24 in one day temporarily | 12 years | 10015D 14467K† | 4397 15702 | 2 4 | 120 |
Symbicort Turbuhaler (DPI) | 100/6 | 2 per day in 1 dose or 2 divided doses | 1 | 6 per occasion 8 per day 12 in one day temporarily | 12 years | 8796Y 14440B† | 4380 15755 | 1 2 | 120 | |
Rilast Turbuhaler Symbicort Turbuhaler (DPI) | 200/6 | 2 per day in 1 dose or 2 divided doses (may increase to 2 twice daily) | 1 | 6 per occasion 8 per day 12 in one day temporarily | 12 years | 8625Y 14439Y† | 7970 15680 | 1 2 | 120 | |
DuoResp Spiromax (DPI) | 200/6 | 2 per day in 1 dose or 2 divided doses (may increase to 2 twice daily) | 1 | 6 per occasion 8 per day 12 in one day temporarily | 18 years | 11273H 14434Q† | 7970 15680 | 1 2 | 120 | |
Bufomix Easyhaler (DPI) | 200/6 | 2 per day in 1 dose or 2 divided doses (may increase to 2 twice daily) | 1 | 6 per occasion 8 per day 12 in one day temporarily | 12 years | 14151T 8625Y 14439Y† | 7970 7970 15680 | 2 1 2 | 60 120 120 | |
Additional information
DPI: dry powder inhaler; ICS: inhaled corticosteroid; pMDI: pressurised metered-dose inhaler
* Inhaled corticosteroid dose-formoterol dose; † Condition must be stable for the prescriber to consider the listed maximum quantity of this medicine suitable for this patient (60-day prescribing); ‡ Maximum doses as stated in TGA-approved product information (daily maximum rarely needed in practice)
Products approved by the Therapeutic Goods Administration for use in maintenance-and-reliever therapy. The patient uses the same inhaler as their regular daily maintenance treatment, and also takes extra doses as anti-inflammatory reliever as needed to manage symptoms. Other combinations of an inhaled corticosteroid and a long-acting beta2 agonist cannot be used this way.
Table
| Treatment strategy | Role |
| Monoclonal antibody therapies (‘biologic’ agents) | Targeted anti-inflammatory treatment according to allergic status and inflammatory phenotype, for patients under specialist care |
| Maintenance high-dose ICS-LABA plus as-needed SABA | Short-term (3–6 months) treatment trial while investigating causes of persistent symptoms/severe exacerbations, or pending eligibility for monoclonal antibody therapy Under specialist care when symptoms and exacerbations cannot be controlled with medium-dose ICS-LABA |
Maintenance ICS-LABA-LAMA plus as-needed SABA (ICS dose medium or high) | Treatment trial in patients with blood eosinophil count/FeNO within normal range, while investigating causes of persistent symptoms/severe exacerbations, or pending eligibility for monoclonal antibody therapy Long-term treatment for selected patients with demonstrated benefit, including those not eligible for monoclonal antibody therapy |
| Montelukast | May be considered as add-on treatment for patients with aspirin-exacerbated respiratory disease Limited use in severe asthma ⚠ Montelukast TGA-approved product information and consumer medicine information carry a warning about potential neuropsychiatric adverse effects. Counsel parents about risks (see TGA safety alert). |
| Azithromycin | An add-on treatment option used in specialist care for patients with persistent exacerbations despite maintenance treatment with medium-dose ICS-LABA. Screening is required and cautions apply (see Centre of Excellence in Severe Asthma guidance on azithromycin). |
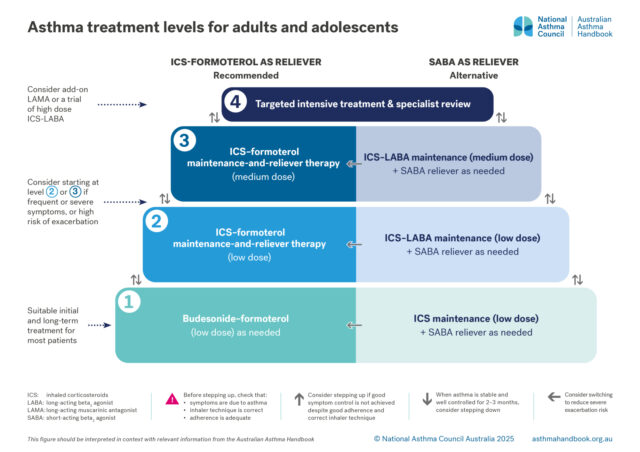
Asthma treatment is adjusted to maintain good control of asthma symptoms and prevent exacerbations, while minimising side-effects. The optimal step for an individual may change over time.
There are four levels of treatment, from least intensive to most intensive.
Level 1. Low-dose budesonide–formoterol, taken as needed (AIR-only)
Level 2. Low-dose MART: maintenance treatment with a low dose of ICS-formoterol, plus extra doses from same inhaler taken as needed for relief of symptoms
Level 3. Medium-dose MART: maintenance treatment with a medium dose of ICS-formoterol (higher number of inhalations using low-dose inhaler), plus extra doses from same inhaler taken as needed for relief of symptoms
Level 4. Targeted intensive treatment – more intensive regimens based on ICS-LABA, sometimes with add-on treatments such as LAMAs or monoclonal antibody therapies.
Level 1: maintenance treatment with a low dose of ICS, plus SABA taken as needed for relief of symptoms
Level 2: maintenance treatment with a low dose of ICS-LABA, plus SABA taken as needed for relief of symptoms
Level 3: maintenance treatment with a medium dose of ICS-LABA, plus SABA taken as needed for relief of symptoms
Treatment regimens with ICS-formoterol as the reliever are recommended:[GINA 2025]
Crossingham I, Turner S, Ramakrishnan S, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev 2021; 5: CD013518.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Murphy J, McSharry J, Hynes L, et al. Prevalence and predictors of adherence to inhaled corticosteroids in young adults (15-30 years) with asthma: a systematic review and meta-analysis. J Asthma 2021; 58: 683-705.
Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193-204.
Rabe KF, Atienza T, Magyar P, et al. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet 2006; 368: 744-753
Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting beta-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A systematic review and meta-analysis. JAMA 2018; 319: 1485-1496.
For recommendations on Level 1 treatment, see: Initial asthma treatment for adults and adolescents
For recommendations on Level 2–3 treatment, see: Adjusting treatment for adults and adolescents
For recommendations on Level 4 treatment, see: Managing difficult-to-treat asthma in adults and adolescents and Specialist assessment and treatment for severe asthma

Guide to asthma medicines, including types of treatment regimens, brand names, single-inhaler combinations, and inhaler…

Medication management
How to choose optimal starting treatment for patients 12 years and over.

Medication management
How to step up or step down asthma treatment for an individual patient to maintain control and minimise side-effects.

Medication management
How to investigate asthma that is not well controlled despite treatment with medium-dose ICS-LABA