Australian Asthma Handbook
The National Guidelines for Health Professionals
Cite
Advise patients to carry their reliever at all times and use it when they experience difficulty breathing, or before anticipated exposure to triggers (e.g. exercise or unavoidable allergen exposure).
Recommendation type: Evidence-based recommendation adopted from GINA
Recommended level 1 treatment: budesonide-formoterol taken as needed for symptom relief
For patients not using maintenance ICS-containing treatment, low-dose budesonide-formoterol taken as needed markedly reduces the risk of severe exacerbations requiring OCS, compared with SABA taken as needed.[O’Byrne 2018, Beasley 2019, Crossingham 2021]
Maintenance ICS treatment is generally not necessary for patients with infrequent symptoms and no specific indications for maintenance ICS treatment, because budesonide-formoterol taken as needed is equally or more effective than daily low-dose ICS for preventing severe exacerbations.[O’Byrne 2018, Bateman 2018, Beasley 2019, Hardy 2019]
Budesonide-formoterol taken as needed results in a lower average ICS dose than daily maintenance ICS.[Beasley 2019, Hardy 2019, O’Byrne 2018, Bateman 2018] Benefits in adolescents are similar to those in adults.[Reddel 2021]
Patients using as-needed low-dose budesonide–formoterol can use this inhaler before exercise, if needed to prevent exercise-induced symptoms.[Lazarinis 2014] They should not use a separate SABA inhaler.
Alternative level 1 treatment option
Maintenance treatment with low-dose ICS (plus salbutamol as needed) is the alternative treatment option for patients with a new diagnosis of asthma.
However, poor adherence to maintenance daily ICS, which is common among patients with infrequent symptoms and minimal experience of severe exacerbations, would result in SABA-only treatment.[GINA 2025] As-needed SABA alone is not recommended for asthma treatment in adults or adolescents.
Bateman ED, Reddel HK, O'Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378: 1877-1887.
Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019; 380: 2020-2030.
Crossingham I, Turner S, Ramakrishnan S, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev 2021; 5: CD013518.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Hardy J, Baggott C, Fingleton J, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019; 394: 919-928.
Lazarinis N, Jørgensen L, Ekström T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax 2014; 69: 130-136.
O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 2018; 378: 1865-1876.
Reddel HK, O'Byrne PM, FitzGerald JM, et al. Efficacy and safety of as-needed budesonide-formoterol in adolescents with mild asthma. J Allergy Clin Immunol Pract 2021; 9: 3069-3077.e3066.
Budesonide–formoterol taken as needed for symptom relief, without maintenance ICS treatment, is sometimes called anti-inflammatory reliever (AIR)-only therapy. Only certain inhalers containing budesonide and formoterol are approved by TGA for this use.
An anti-inflammatory reliever is an ICS-formoterol combination approved by TGA for as-needed use, either alone (certain inhalers containing budesonide-formoterol) or in addition to maintenance treatment with the same inhaler (certain inhalers containing budesonide-formoterol and certain inhalers containing beclometasone-formoterol).
Recommendation type: Evidence-based recommendation adopted from GINA
Early initiation of ICS treatment after diagnosis of asthma improves lung function, compared with no ICS for 2–4 years.[Selroos 1995, Busse 2008]
The use of SABA as needed, without ICS treatment, increases the risk of exacerbations compared with budesonide-formoterol taken as needed, even in patients with infrequent symptoms.[Beasley 2019] Self-adjustment of the budesonide-formoterol dose by taking extra doses immediately in response to recurring symptoms appears to reduce the short-term risk of a severe exacerbation, compared with using SABA.[O’Byrne 2021]
SABA-only treatment is associated with a higher risk of severe asthma exacerbations, compared with maintenance ICS treatment. [Suissa 2002]
Over-use of SABA (dispensing of 3 or more 200-dose canisters in one year) is associated with increased risk of severe exacerbations. [Stanford 2012, Nwaru 2020] Dispensing of 12 or more canisters in one year is associated with increased risk of asthma death. [Nwaru 2020, Suissa 1994]
Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019; 380: 2020-2030.
Busse WW, Pedersen S, Pauwels RA, et al. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121: 1167-1174.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Nwaru BI, Ekstrom M, Hasvold P, et al. Overuse of short-acting beta2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J 2020; 55: 1901872.
O'Byrne PM, FitzGerald JM, Bateman ED, et al. Effect of a single day of increased as-needed budesonide-formoterol use on short-term risk of severe exacerbations in patients with mild asthma: a post-hoc analysis of the SYGMA 1 study. Lancet Respir Med 2021; 9: 149-158.
Selroos O, Pietinalho A, Lofroos AB, et al. Effect of early vs late intervention with inhaled corticosteroids in asthma. Chest 1995; 108: 1228-1234.
Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med 1994; 149: 604-610.
Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax 2002; 57: 880-884.
Stanford RH, Shah MB, D’Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol 2012; 109: 403-407.
Recommendation type: adapted from GINA
The use of a pMDI with spacer delivers inhaled asthma medicines to the lungs more quickly and at least as effectively as a nebuliser.[Newman 2002]
The association between SABA use and increased risk of exacerbations is stronger for nebulized salbutamol than salbutamol delivered by pMDI.[Paris 2008]
The use of nebulisers is unnecessary except in some cases of severe acute asthma. In adults, the use of nebulisers for SABA is associated with a higher risk of exacerbations than the use of inhalers.[Paris 2008]
The use of nebulisers may increase the risk of viral transmission.[Hui 2009, Biney 2024, Goldstein 2021] Healthcare workers should follow infection control procedures including use of personal protective equipment such as face masks.
Biney IN, Ari A, Barjaktarevic IZ, et al. Guidance on mitigating the risk of transmitting respiratory infections during nebulization by the COPD Foundation Nebulizer Consortium. Chest 2024; 165: 653-668.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Goldstein KM, Ghadimi K, Mystakelis H, et al. Risk of transmitting coronavirus disease 2019 during nebulizer treatment: a systematic review. J Aerosol Med Pulm Drug Deliv 2021; 34: 155-170.
Hui DS, Chow BK, Chu LC, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 2009; 135: 648-654.
Newman KB, Milne S, Hamilton C, Hall K. A comparison of albuterol administered by metered-dose inhaler and spacer with albuterol by nebulizer in adults presenting to an urban emergency department with acute asthma. Chest 2002; 121: 1036-1041.
Paris J, Peterson EL, Wells K, Pladevall M, Burchard EG, Choudhry S, Lanfear DE, Williams LK. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol 2008; 101: 482-487.
More information on selecting inhalers for adults & adolescents
More information on inhalers in the Medicines guide
For these patients, consider starting with MART (low or medium dose, according to risk assessment), or maintenance ICS-LABA (low or medium dose) plus SABA as needed.
This recommendation applies to patients with frequent or severe symptoms at the time of diagnosis, a known asthma exacerbation (or an episode of acute respiratory signs and symptoms) that required treatment with systemic corticosteroids any time in the previous 12 months, or a lifetime history of a life-threatening asthma exacerbation or acute respiratory episode.
Recommendation type: adapted from GINA
MART is recommended as initial treatment for patients with frequent symptoms or a history of a severe asthma exacerbation because it effectively manages the risk of exacerbations and ensures the patient receives an ICS dose proportional to the frequency of symptoms. Alternative options include maintenance treatment with a low or medium dose of ICS-LABA, plus SABA as needed for symptom relief.
MART reduces the rate of exacerbations compared with fixed-dose maintenance ICS-LABA regimens (with SABA as needed) at the same or a higher ICS dose.[Sobieraj 2018]
Low-dose MART is associated with a reduction in severe exacerbations and a similar level of control, at relatively low ICS doses, compared with maintenance ICS-LABA plus as-needed SABA or a higher dose of ICS plus as-needed SABA.[Cates 2013, Kew 2013, Papi 2013, Patel 2013, Bateman 2011, Jorup 2018]
The benefit of the MART regimen in reducing the risk of severe exacerbations requiring OCS appears to be due to the increase in doses of both the ICS and the formoterol at a very early stage of worsening asthma.[GINA 2025]
Bateman ED, Harrison TW, Quirce S, et al. Overall asthma control achieved with budesonide/formoterol maintenance and reliever therapy for patients on different treatment steps. Respiratory res 2011; 12: 38.
Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev 2013; 4: CD007313.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Jorup C, Lythgoe D, Bisgaard H. Budesonide/formoterol maintenance and reliever therapy in adolescent patients with asthma. Eur Respir J 2018; 51: 1701688.
Kew KM, Karner C, Mindus SM, et al. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev 2013; 12: CD009019.
Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide/formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med 2013; 1: 32-42.
Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone–formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med 2013; 1: 23-31.
Sobieraj DM, Weeda ER, Nguyen E, et al. Association of inhaled corticosteroids and long-acting beta-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A systematic review and meta-analysis. JAMA 2018; 319: 1485-1496.
Previous asthma exacerbations might include suspicious episodes of acute respiratory disease occurring prior to the diagnosis of asthma (e.g. episodes recorded as bronchitis, acute respiratory infection or nonspecific events).
Recommendation type: Evidence-based recommendation adopted from GINA
MART is recommended as initial treatment for patients with frequent symptoms or a recent severe asthma exacerbation as a strategy to gain rapid control over symptoms and reduce the risk of exacerbations, while adjusting the dose to the patient’s symptoms.
Alternative options include maintenance treatment with a low or medium dose of ICS-LABA, plus SABA as needed for symptom relief.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, 2025. Available from: www.ginasthma.org
Scenarios in which an adult or adolescent without known asthma might present to general practice or an emergency department with acute respiratory symptoms include a first episode of thunderstorm asthma, or acute bronchoconstriction triggered by medicines (including NSAID ingestion in a person with undiagnosed aspirin-exacerbated respiratory disease).
More information on thunderstorm asthma
More information on triggers
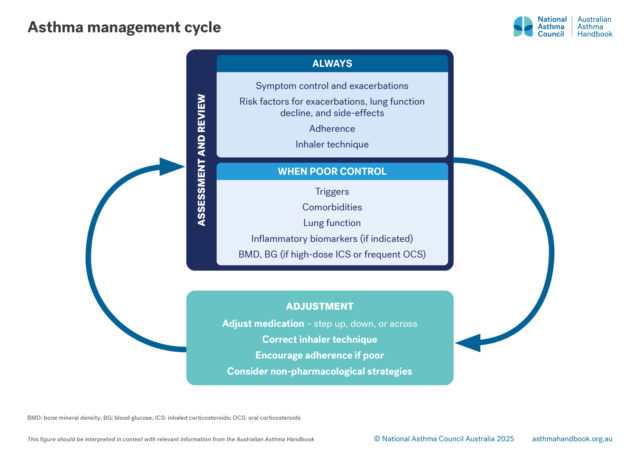
Principles of management
How to assess asthma control and severity. Risk factors for asthma exacerbations and what to include at check-ups for…
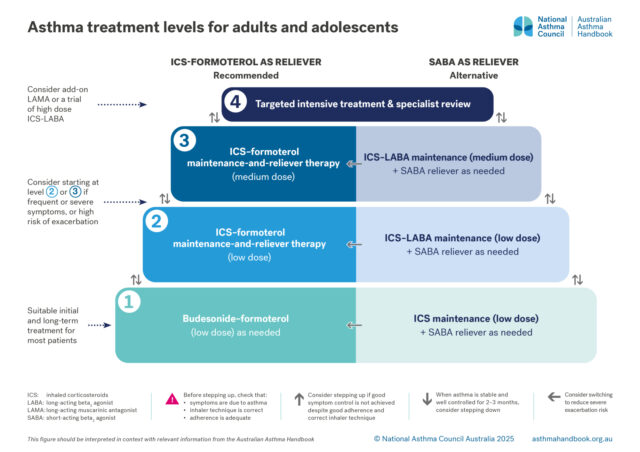
Medication management
Recommended and alternative treatment options for patients 12 years and over, according to intensity of treatment…

Guide to asthma medicines, including types of treatment regimens, brand names, single-inhaler combinations, and inhaler…

Principles of management
How to equip and coach patients to manage their own asthma, including exacerbations.

Adults and Adolescents
How to conduct a rapid assessment and administer bronchodilators and supplementary oxygen to patients aged 12+ years…