Australian Asthma Handbook
The National Guidelines for Health Professionals
In Australia an estimated 12–13% of pregnant women have asthma.
Good asthma symptom control and prevention of exacerbations protects the foetus as well as the mother.
As with non-pregnant adults, the aim of asthma management during pregnancy is to maintain the best possible asthma control and to avoid asthma exacerbations.
During pregnancy treatment can be stepped up as necessary to regain or maintain control.
Asthma treatment should not be stepped down during pregnancy unless the dose of one or more medicines is inappropriately high.
Australian information and guidance on the safety of asthma medicines during pregnancy
Thoracic Society of Australia and New Zealand (TSANZ) joint statement with the European Respiratory Society (ERS) Task Force Statement on the management of reproduction and pregnancy in women with airways diseases
Therapeutic Goods Administration (TGA): Prescribing medicines in pregnancy database
In Australia an estimated 12–13% of pregnant women have asthma.[Murphy 2023]
Good asthma control during pregnancy is a high priority, to protect the foetus as well as the mother. Untreated asthma, poorly controlled asthma or exacerbations during pregnancy put mothers and babies at risk.
Reducing asthma-related risk for women with asthma and their babies involves:
As with any medication required during pregnancy, the benefits and risks of treatment for both the patient and the foetus must be considered. In general, the risks of poor asthma control outweigh the risks associated with medicines.
Most asthma medicines can be used by breastfeeding women; prescribers should check TGA-approved product information.
Asthma has been associated with reduced fertility in women: increased time to pregnancy and reduced birth rate.[Middleton 2020] Despite this, asthma in pregnancy is common.[Murphy 2023]
If a patient without a previous diagnosis of asthma presents with respiratory symptoms during pregnancy, these must be distinguished from normal physiological changes. An estimated 60%–70% of pregnant women experience dyspnoea during the first and second trimesters.[Bravo-Solarte 2023] Shortness of breath that impairs functionality and is associated with other symptoms, such as cough or wheeze, should be investigated.
Pregnancy affects lung function: expiratory reserve and residual volumes decrease, while tidal volume increases. However, there is no change in forced vital capacity or peak expiratory flow.[Bravo-Solarte 2023] Lung function changes are more pronounced in pregnant women with asthma than those without.[Bravo-Solarte 2023]
Bronchial provocation testing is contraindicated during pregnancy.[GINA 2025]
Approximately 40% of pregnant women with asthma experience worsening asthma symptoms, and at least 20% have an exacerbation that requires medical intervention.[Murphy 2023] Worsening symptoms and exacerbations are most common during the second trimester. [Murphy 2023]
Asthma exacerbations during pregnancy are associated with low birth weight, preterm birth, and small for gestational age status.[Murphy 2023] However, among women with asthma that is managed by a health professional, the risk of preterm labour and preterm delivery is not significantly higher than for pregnant women without asthma.[Murphy 2011]
Comorbid conditions that are common in pregnancy may affect asthma control or mimic asthma symptoms (e.g. allergic rhinitis, gastro-oesophageal reflux disease).
As for all adults and adolescents, asthma during pregnancy should be managed with the aim of maintaining the best possible asthma control and avoiding asthma exacerbations. The treatment regimen should be stepped up as necessary to regain or maintain control during pregnancy. Asthma treatment should not be stepped down during pregnancy unless the dose of one or more medicines is inappropriately high.
Asthma management in pregnancy includes control of comorbidities such as rhinitis, obesity, snoring and sleep-disordered breathing, and advising pregnant women to avoid cigarette smoke and viral infections.[Middleton 2020]
As with any medication required during pregnancy, the benefits and risks of treatment for both the patient and the foetus must be considered. In general, the risks of poor asthma control outweigh the risks associated with medicines.
Most asthma medicines can be used by breastfeeding women; for most medicines for which safety categories are available, expert panels have either concluded that levels in breastmilk are negligible, or assessed the risks of poor asthma control to the mother to outweigh any risk to the baby associated with medicines in breastmilk.
Safety data are limited for many medicines. In general, more experience with use during pregnancy has been documented for older medicines.
Table
Class | Source | ||
TGA* | ERS/TSANZ task force | ||
| Short-acting beta2 agonists | Overall | – | Unlikely to cause structural anomalies Avoid excessive use |
| Salbutamol | Category A | Compatible (all) | |
| Terbutaline | Category A | Probably safe (all) | |
| Long-acting beta2 agonists | Overall | – | Limited data from humans and animals suggest no risk or low risk salmeterol and formoterol |
| Formoterol | Category B3 | Probably safe (all) Acceptable to continue if symptoms were already controlled with formoterol treatment before conception | |
| Indacaterol | Category B3 | – | |
| Salmeterol | Category B3 Benefits outweigh risk | Probably safe (all) Greater experience of use in pregnancy | |
| Vilanterol | Category B3 | – | |
| Long-acting muscarinic antagonists | Glycopyrronium | Category B2 | – |
| Tiotropium | Category B1 | Compatible (breastfeeding) Probably safe (pregnancy and labour) | |
| Umeclidinium | Category B1 | – | |
| Inhaled corticosteroids | Overall | – | At usual doses, ICSs have not been associated with increased risk of major malformations, intrauterine growth restriction, pre-term delivery or low birthweight Use lowest dose necessary to maintain asthma control Greater volume of pregnancy safety data available for budesonide and beclometasone Any ICS can be continued if asthma was controlled during treatment before conception |
| Beclometasone | Category B3 Benefits outweigh risk | Compatible (all) Beclometasone >1000 microg/day associated with a small risk of congenital malformation in one study | |
| Budesonide | Category A Benefits outweigh risk | Compatible (all) | |
| Fluticasone furoate | Category B3 Benefits outweigh risk | ||
| Ciclesonide | Category B3 | Probably safe (all) | |
| Fluticasone propionate | Category B3 Benefits outweigh risk | Compatible (all) | |
| Mometasone furoate | – | Probably safe (all) | |
| Monoclonal antibody therapy | Overall | – | Very limited evidence |
| Benralizumab | Category B1 | Possibly safe (all) | |
| Dupilumab | Category B1 | Possibly safe (all) | |
| Mepolizumab | Category B1 | Possibly safe (all) | |
| Omalizumab | Category B1 | Probably safe (pregnancy) Possibly safe (labour and breastfeeding) | |
| Leukotriene receptor antagonists | Montelukast | Category B1 | Probably safe (all) |
| Systemic corticosteroids | Prednisolone/prednisone | Category A | Possibly safe (all) Conflicting safety data |
| Intranasal corticosteroids | – | As for inhaled formulations with same molecule | Compatible (breastfeeding) Probably safe (pregnancy and labour) |
| Allergen immunotherapy | – | – | Recommendation against initiating allergen immunotherapy during pregnancy Can be continued during pregnancy if well tolerated before pregnancy |
Additional information
TGA: TGA. Prescribing medicines in pregnancy database. The Australian categorisation system and database for prescribing medicines in pregnancy. [Website] [Accessed February 2025] Australian Government Department of Health and Aged Care Therapeutic Goods Administration.
ERS/TSANZ task force: Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55: 1901208.
* Check latest assessment on the TGA database of medicines rated under the Australian categorisation system for prescribing medicines in pregnancy
Category A: Australian categorisation system for prescribing medicines in pregnancy assessment: ‘Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed.’ [TGA]
Category B: Australian categorisation system for prescribing medicines in pregnancy assessment: ‘Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have not shown evidence of an increased occurrence of fetal damage.’ [TGA]
Category B3: Australian categorisation system for prescribing medicines in pregnancy assessment: ‘Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans.’ [TGA]
Benefits outweigh risk: TGA statement ‘The benefits of asthma control outweigh any potential for an adverse pregnancy outcome’ applies. [TGA]
Compatible (all): ERS/TSANZ task force judgment ‘compatible with all stages of pregnancy, labour, and breastfeeding’. In general, a first-choice option; sufficient anecdotal evidence of very low or non-existent risk to embryo or foetus, based on use during human pregnancies. [Middleton 2020]
Compatible (specified): ERS/TSANZ task force judgment ‘compatible with’ specified stage (pregnancy, labour, or breastfeeding) [Middleton 2020]
Probably safe (all): ERS/TSANZ task force judgment ‘probably safe throughout all stages of pregnancy, labour, and breastfeeding’. In general, characteristics of the medicine or medicines in the same class suggest low risk, but limited trial experience during human pregnancy or breastfeeding. [Middleton 2020]
Probably safe (specified): ERS/TSANZ task force judgment ‘probably safe’ during specified stage [Middleton 2020]
Possibly safe (all): ERS/TSANZ task force judgment ‘possibly safe during all stages of pregnancy, labour, and breastfeeding.’ Considered for second-line use if better-tested treatment options fail. Direct maternal benefit is thought likely to outweigh potential risk during pregnancy and/or breastfeeding, but exact risks are unknown. [Middleton 2020]
Possibly safe (specified): ERS/TSANZ task force judgment ‘possibly safe’ during specified stage. [Middleton 2020]
The TGA maintains a database of safety categories for medicines during pregnancy.[TGA]
The ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases assesses the safety of asthma medicines during pregnancy, labour and breastfeeding as one of three categories:[Middleton 2020]
A 2025 international consensus statement on the use of monoclonal antibody therapy (biologics) for asthma during pregnancy based its conclusions on available evidence for harms associated with uncontrolled asthma, and harms associated with asthma treatments during pregnancy from observational studies including registry databases, and post-marketing surveillance studies.[Naftel 2025]
TGA’s Prescribing medicines in pregnancy database
ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases
Naftel J, Jackson DJ, Coleman M, et al. An international consensus on the use of asthma biologics in pregnancy. Lancet Respir Med. 2025; 13: 80-91.
There is very limited evidence for the efficacy and safety of monoclonal antibody therapy for asthma during pregnancy, because pregnant women were excluded from clinical trials.[Naftel 2025] An ERS/TSANZ Task Force stated that monoclonal antibodies were unlikely to cross the placenta in sufficient quantities to cause foetal harm and advised that monoclonal antibody therapy should be continued during pregnancy if required for asthma control in the mother.[Middleton 2020]
A 2025 international consensus statement [Naftel 2025] advised that:
Acute asthma in a pregnant patient should be treated promptly to minimise risk to the foetus as well as the woman.
It should be managed as for all adults and adolescents, including with oral corticosteroids if indicated.
Exacerbations of asthma are uncommon during labour.[Middleton 2020]
There is no published evidence that inhaled beta2 agonists affect the course of labour.[Middleton 2020]
Neither the use of prostaglandin E2 for induction of labour, nor the use of oxytocin for augmentation of the second and third stages of labour, have been associated with worsening lung function or asthma exacerbations.[Middleton 2020]
The ERS/TSANZ statement recommends oxytocin as the preferred uterotonic agent for the active third stage of labour because ergotamine may cause bronchospasm, particularly in association with general anaesthesia.[Middleton 2020]
Bravo-Solarte DC, Garcia-Guaqueta DP, Chiarella SE. Asthma in pregnancy. Allergy Asthma Proc 2023; 44: 24-34.
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2025. Available from: www.ginasthma.org
Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J 2020; 55: 1901208.
Murphy VE, Gibson PG, Schatz M. Managing asthma during pregnancy and the postpartum period. J Allergy Clin Immunol Pract 2023; 11: 3585-3594.
Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118: 1314-1323.
Naftel J, Jackson DJ, Coleman M, et al. An international consensus on the use of asthma biologics in pregnancy. Lancet Respir Med 2025; 13: 80-91.
TGA. Prescribing medicines in pregnancy database. The Australian categorisation system and database for prescribing medicines in pregnancy. [Website] [Accessed February 2025] Australian Government Department of Health and Aged Care Therapeutic Goods Administration.
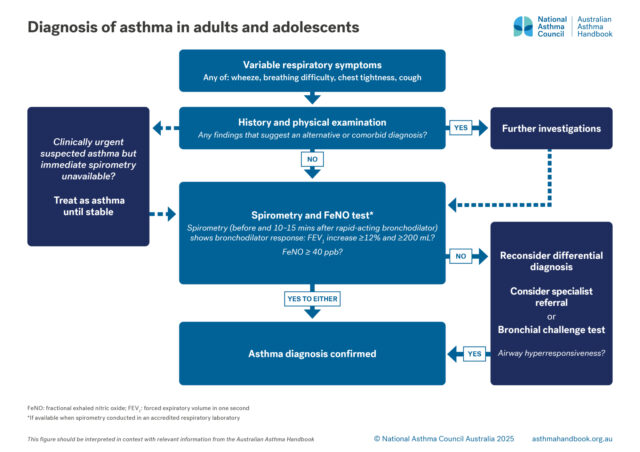
Adults and Adolescents
Investigation of suspected asthma and diagnostic criteria in adolescents and adolescents.

Principles of management
Goals and principles of asthma management in adults and adolescents.

Medication management
How to step up or step down asthma treatment for an individual patient to maintain control and minimise side-effects.

Clinical topics
Information on risk factors for developing asthma, and on evidence for prevention strategies.