Australian Asthma Handbook
The National Guidelines for Health Professionals
Principles of asthma management, guidance on prescribing medicines, and holistic asthma care for primary school children.

Principles of management
Goals and principles of asthma management in primary school-aged children.

Principles of management
How to assess asthma control and severity in children. Risk factors for asthma exacerbations and what to includes at…

Principles of management
How to administer inhaled medicines to children.

Principles of management
How to equip and coach children and their parents to manage childhood asthma, including exacerbations.

Medication management
How to choose optimal starting treatment for children aged 6–11 years.
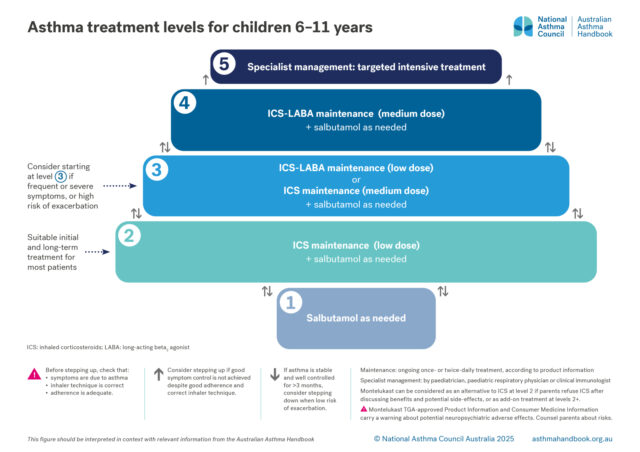
Medication management
Treatment options for primary school-aged children, classified according to the intensity of treatment needed to…

Medication management
How to step up or step down asthma treatment for a child aged 6–11 years, to maintain symptom control, prevent…
Medication management
How to manage a child’s mild asthma exacerbations that do not require an ED visit.

Medication management
How to investigate asthma that is not well controlled despite treatment with medium-dose ICS-LABA.

Medication management
Overview of specialist management of severe asthma in patients aged 6–11 years. Practical advice for primary care…

Adjunctive management
What to advise parents about avoiding exposures that trigger their child’s asthma.

Adjunctive management
When to consider allergen immunotherapy for children 6–11 years with allergic asthma.

Adjunctive management
Advising parents about exercise and diet.