Australian Asthma Handbook
The National Guidelines for Health Professionals
The basics of asthma care for people 12 years and over.

Principles of management
Goals and principles of asthma management in adults and adolescents.
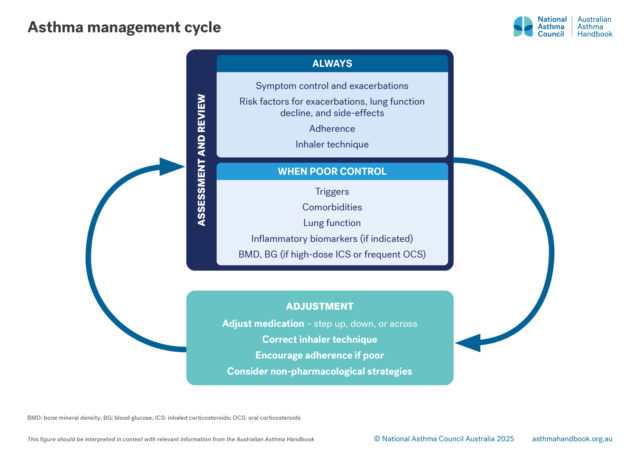
Principles of management
How to assess asthma control and severity. Risk factors for asthma exacerbations and what to include at check-ups for…

Principles of management
How to choose and use asthma inhalers for adults and adolescents.

Principles of management
How to equip and coach patients to manage their own asthma, including exacerbations.