Australian Asthma Handbook
The National Guidelines for Health Professionals
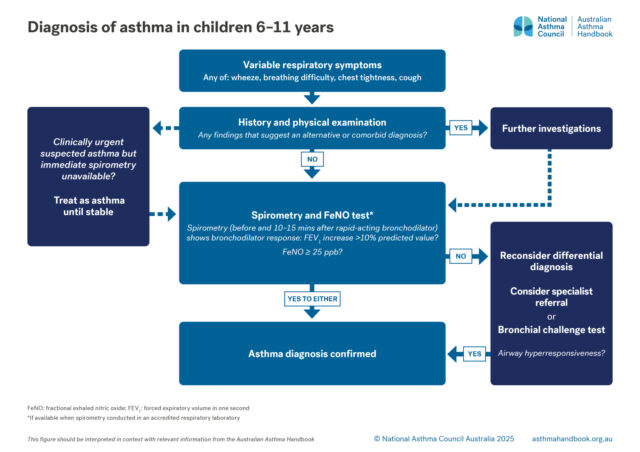
Definition of asthma and guidance on diagnosing asthma in primary school children.

Children 6-11 years
Asthma is a chronic inflammatory lung condition, clinically defined as the combination of variable respiratory symptoms…
Children 6-11 years
Investigation of suspected asthma and diagnostic criteria in children 6–11.